Which of the Following Best Describes an Empirical Formula
Which of the following statements correctly describe the empirical formula for a compound. Determine the empirical formula of a compound containing 406 grams of carbon 51 grams of hydrogen and 542 grams of oxygen.

Molecular Formulas And Nomenclature
Correct option is C Empirical formula is the simplest ratio of molecules present in a compound.

. The empirical formula shows or describes the simplest whole number ratio of all the atoms present in a molecule. Is the simplest whole number ratio of each type of atom in a compound. What is the empirical formula if you have 3684 nitrogen and 6316 oxygen.
What is the empirical formula if you have 3598 aluminum and 6402 sulfur. Updated on July 03 2019. Learn about our Editorial Process.
The empirical formula of a compound represents the simplest whole-number ratio between the elements that make up the compound. Empirical formula of C₂H₆O₂ CH₃O So Option C is correct. The first sample contains 1 mole of iron Fe and the second sample contains 1 mole of lithium Li.
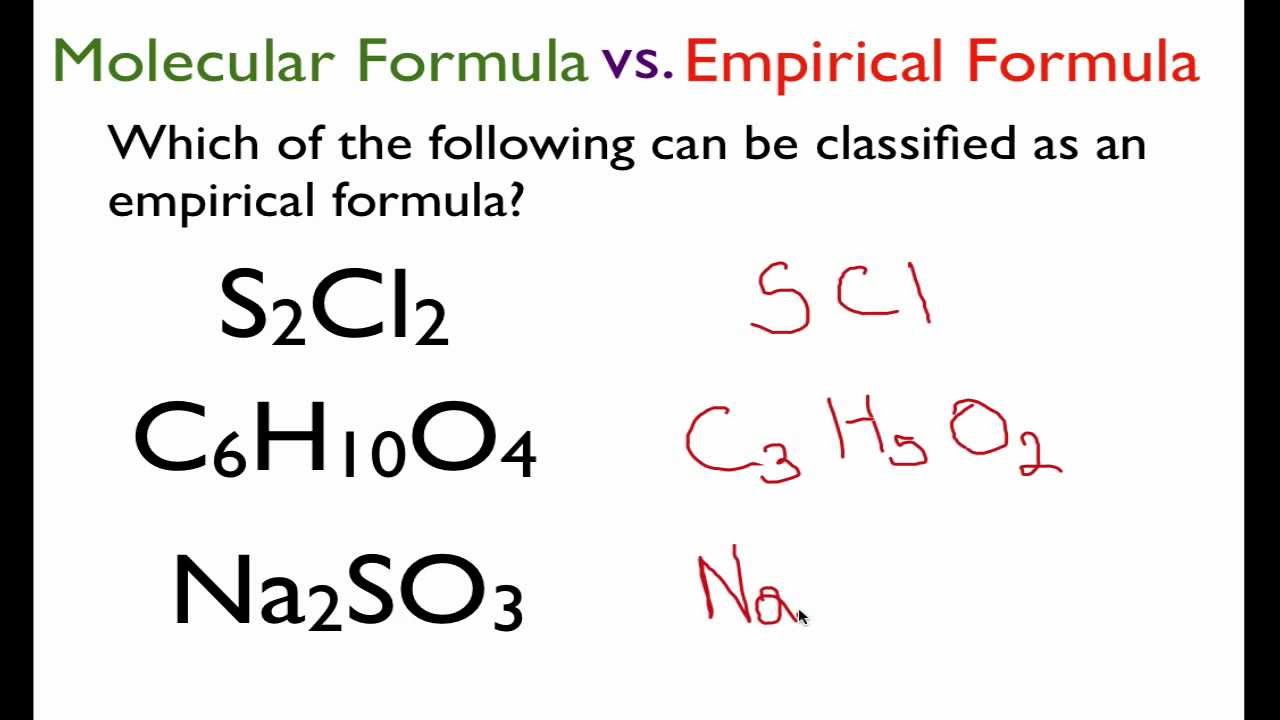
The empirical formula is the simplest whole-number ratio of atoms in a compound. Different compounds can have the same empirical formula. Which of the following is an example of an empirical formula.
Now you have the subscripts for the atoms in the empirical formula. Students are given two samples of material. Select all that apply.
Question 1 1 point Q25 Which of the following is the empirical formula of a compound that is 273 mass percent carbon and 727 mass percent oxygen. Which of the following statements best describes how these samples compare to one another. For example the empirical formula of ethane is CH3 because both numbers in its molecular formula can be simplified by dividing by 2.
__Al2S3s __H2Ol __AlOH3aq __H2Sg The molecular formula of caffeine is C8H10N4O2. A C20 B CO3 C C202 D C308 E CO2 Question 2 1 point Q26 A compound containing only silicon and chlorine is 791 mass percent chlorine. What is the molecular formula of the compound.
However one structural representation for butane is CH 3 CH 2 CH 2 CH 3 while isobutane can be described using the structural formula CH 3 3 CH. Its empirical formula is _____. Empirical formula C 6 H 11 NO.
CaO 2 H 2. K is the thermal conductivity. 0675 mol O 0337 200 mol O.
Which of the following best describes the trajectory of a projectile. Empirical formula is same as molecular mass as n1 this means molecular formula is. A periodic table will be required to complete this practice test.
Empirical formulas are the simplest types of chemical formulas that indicate the ratio of each element in the molecule. Which of the following best describes the change that takes place immediately after the CH3OH l is introduced into the previously evacuated vessel. For butane and isobutane the empirical formula for both molecules is C 2 H 5 and they share the same molecular formula C 4 H 10.
For example the empirical formula of glucose is CH2O while the molecular formula is. An empirical formula is a chemical formula that has simplest whole number ratios on atoms. Which of the Following Best Describes an Empirical Formula By Bio_Presley407 13 Apr 2022 Post a Comment.
Cg HigCl₂ O c. Start studying the exam 5 flashcards containing study terms like Balance the illustrated chemical equation by matching each substance to the correct coefficient. In an experiment the molar mass of the compound was determined to be 118084 gmol.
A chemical formula that shows the relative number of each type of atom in. Therefore if we look at the subscript numbers in an empirical formula we should not be able to divide them anymore. If you react 3406 g NH3 with 6400 g O2 which of the following statements best describes the limiting reactant situation of this reaction.
0668 mol H 0337 198 mol H which rounds up to 200. The empirical formula of a compound is COCl 2 and its molecular mass is 9000u. This 10-question practice test deals with finding empirical formulas of chemical compounds.
A chemical formula that shows the relative number of each type of atom in a molecule using the smallest possible ratio - Apex. Chemistry questions and answers. A Molecular oxygen is the.
Find out the molecular formula of that compound. Lets look at each answer choice and determine whether it is an empirical formula or not. 2 NaHCO3s Na2CO3s CO2g H2Og Which of the following describes the changes in forces of attraction that occur as H2O changes phase from a liquid to a vapor.
Memorize flashcards and build a practice test to quiz yourself before your exam. Finally apply the rules of writing formulas to present the formula correctly. Calculate the empirical formula.
4207 Na 1889 P and 3904 O determine the empirical formula. Sample 1 contains more atoms than sample 2. A the height of the shooter b the path of the flight of a bullet c the housing for the bullets.
Answer and explanation. So in an empirical formula ratio of molecules cannot have a common factor. For both questions show your work or explain how you determined.
Fe2O3 Al -- Al2O3 Fe unbalanced If you have 30 g of Fe2O3 and 30 g of Al which is the limiting reagent. Hence the key difference between empirical and molecular formulas is that empirical formula only gives the simplest ratio of atom whereas molecular. The cation of the compound is written first followed by the anion.
Empirical formulas are the simplest form of formulas that we can write for a molecule while molecular formulas are the formulas showing the type of atoms and number of each atom connected in the molecule. COCl 2 C O 2Cl 12 16 2355 99 u. Which of the following is the most likely empirical formula of a compound formed from element X and phosphorus P.

Classic Chemistry Finding The Empirical Formula Science In School
Chemical Formulas Boundless Chemistry

What Is Empirical Formula Give An Example For A Compound Whose Empirical Formula And Molecular Formula Are Same Give An Example

Empirical Formula Definition Steps Examples Video Lesson Transcript Study Com
No comments for "Which of the Following Best Describes an Empirical Formula"
Post a Comment